Salt, the Only Rock We Eat! (May 2025)
Henry Shaw, UC Master Food Preserver Online Program Volunteer
Part 1: When is a tablespoon not a tablespoon?
Common salt, sodium chloride (NaCl), is the most widely used condiment worldwide. It’s also the only rock we normally eat. (“Halite” is the geological term for rock salt.) Today, you can buy a pound of salt for a dollar or so, but a few centuries ago, salt was literally worth its weight in gold (extended history down below).
In food preservation recipes, salt can play two different roles. Often, it’s simply a flavoring agent, in which case the amount of salt in a recipe can safely be adjusted to suit one’s personal preferences. For some techniques, though, the quantity of salt used in a recipe or process is critical to the safety and quality of the final product. Prime examples of when the salt content is important are recipes for lacto-fermented products like fermented pickles, sauerkraut, and kimchi. In the lacto-fermentation process, beneficial lactic-acid bacteria (primarily Lactobacillus species), convert the sugars in food to lactic acid, which lowers the pH and creates a characteristic sour flavor. The resulting acidity of the final product is important for the long-term preservation of the product and for suppressing the growth of Clostridium botulinum (the bacterium that causes botulism).
How do we encourage the growth of desirable bacteria and suppress the growth of undesirable bacteria and molds during fermentation? If you’ve read this far, then you might guess that salt is involved, and it absolutely is! Adding salt to your ferment is how we “rig the game” to help lactic-acid bacteria win the microbial race against the growth of undesirable microorganisms during fermentation. At salt concentrations between 2% – 5% by weight, lactic acid bacteria grow more quickly than other microbes and have a competitive advantage. At lower concentrations, undesirable bacteria can survive/thrive, possibly out-competing lactic-acid bacteria and spoiling your ferment. Too much salt will suppress the growth of lactic-acid bacteria, leaving your vegetables unpickled. Furthermore, salt-tolerant yeasts can grow more quickly, consuming lactic acid and making the product less acidic and more hospitable to spoilage microbes.
Unfortunately, most fermentation recipes specify the amount of salt to use in terms of volume (i.e., X tablespoons of salt per quart of brine or pounds of produce). The problem with this is that there are many different types of salt, and the size and shape of individual particles in these salts can vary dramatically. These microscopic differences in the texture of the salt change the “packing density” of the salt particles, causing the weight (mass) of salt per tablespoon to also vary dramatically. Ideally, the quantity of salt and all other recipe ingredients would be given in terms of weight, which would eliminate the uncertainty in how much salt to use. If a recipe uses volume to specify the amount of salt it should also specify the type of salt to use. If it does not, use a salt designed for canning and pickling. If a specific type of salt is specified, be sure to follow the recipe and use that type of salt.
My wife and I like to cook so we have collected many different salts in our pantry. For fun, I compared the weight of a tablespoon of the eight different salts we have on hand. I also took microphotographs of these salts to illustrate the large differences in particle size and shape in this sample of the “salt universe”. I used a 2-tablespoon measure to scoop each salt from its container and leveled the measure with the back of a knife. The weight of each salt was measured with a calibrated kitchen scale that’s accurate to ±0.02 g. The resulting weights were divided by two to get the weight per tablespoon shown in Figure 1 and tabulated in Table 1, graphed in Figure 2.
I was surprised by the magnitude of the variation in bulk densities of the salts I measured. Morton Canning & Pickling Salt was the densest, weighing in at 18.9 grams/Tbs. while Diamond Crystal Kosher Salt Flakes was the “fluffiest” and least dense, weighing in at 8.2 grams/Tbs. That’s a factor of 2.3 difference; one would need to use 2.3 tablespoons of the Diamond Crystal Flake salt to equal the mass of 1 tablespoon of the canning salt! If I were following a standard recipe that says to use 3 Tbs. of canning salt per 5 pounds of cabbage in a sauerkraut recipe (aiming at adding 2.25 – 2.50% salt) and I used 3 Tbs. of the Diamond Crystal Flake salt instead of the canning salt, the mixture would not have nearly enough salt to ferment properly and it would probably end up a rotten mess. I would have needed to add 6.9 Tbs. (almost ½ cup) of the flake salt to have added enough!
The microphotographs in Figure 1 tell the story; the canning salt consists of very uniform, ~0.5 mm rounded cubes while the two “flake” salts (Penzey’s and Diamond Crystal) have larger, highly irregular particles. The uniform and smooth-sided particles of canning salt can pack more closely. In contrast, the irregular, jagged flakes of the flake salts prevent the close packing of the individual salt particles and leads to a less-dense salt. Our usual “table salt”, the Diamond Crystal Granulated Salt, had a density about 10% less than that of the canning salt, so it would be a reasonable substitute for canning salt. Its particles are more irregularly shaped than the canning salt and they have more variation in particle size, but their rounded shapes allow them to pack at a relatively high density. The average particle size does not seem to be the primary factor determining the bulk density of salt; the shape of the particles is more important. Flaky, irregular particles pack more loosely leading to lower bulk densities.
Figure 1. Macro- and microphotographs of various commercially available salts. The white scale bar in the microphotographs is 3 mm. long. The weight of salt in a tablespoon of salt is given below each salt type and in Table 1.

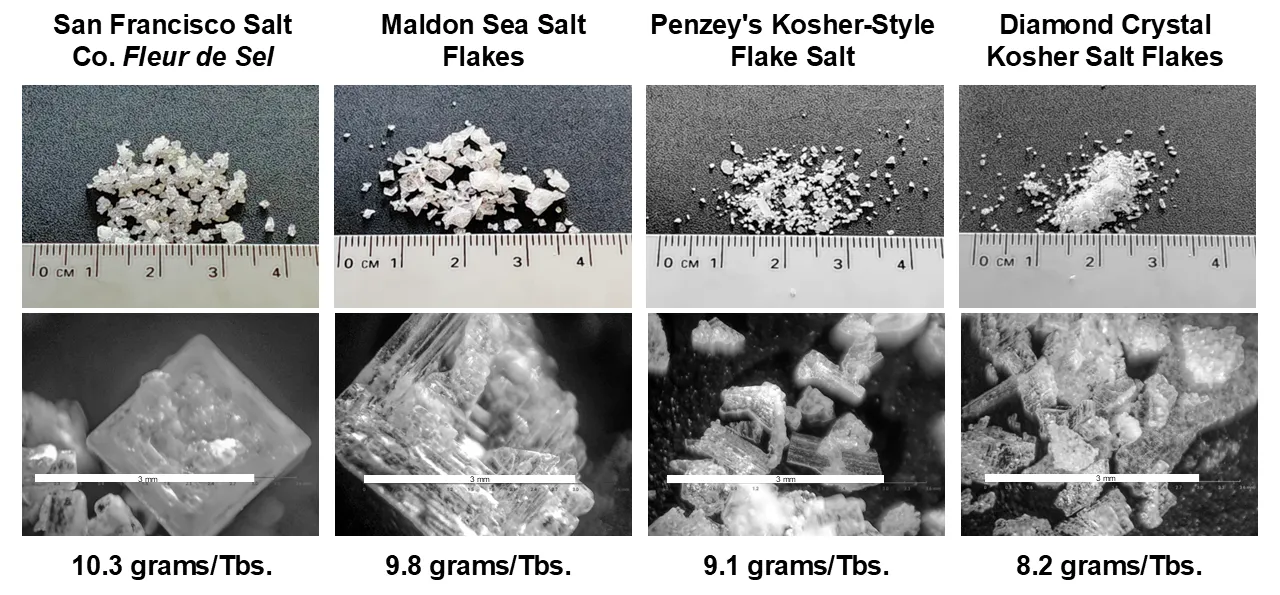
Table 1. Characteristics of various commercial salts
| Salt Name | Typical particle size | grams/Tbs. | Tbs./ounce | Tbs. of salt needed to equal the weight of 1 Tbs. of Morton canning salt |
| Morton Canning & Pickling Salt | ~0.5 mm | 18.9 | 1.5 | 1 |
| Diamond Crystal Granulated Salt (uniodized) | 0.3 - 1.5 mm | 16.9 | 1.7 | 1.1 |
| Baleine Fine Sea Salt | 0.5 - 1.5 mm | 15.8 | 1.8 | 1.2 |
| Kirkland Pure Sea Salt | 0.3 - 0.6 mm | 14.9 | 1.9 | 1.3 |
| San Francisco Salt Co. Fleur de Sel | 0.5 - 5.0 mm | 10.3 | 2.8 | 1.8 |
| Maldon Sea Salt Flakes | 1.0 - 7.0 mm | 9.8 | 2.9 | 1.9 |
| Penzey's Kosher Style Flake Salt | 0.5 - 1.5 mm | 9.1 | 3.1 | 2.1 |
| Diamond Crystal Kosher Salt Flakes | 0.5 - 1.5 mm | 8.2 | 3.5 | 2.3 |
Figure 2. Bar graph showing the bulk density (grams of salt per tablespoon) of the salts listed in Table 1.
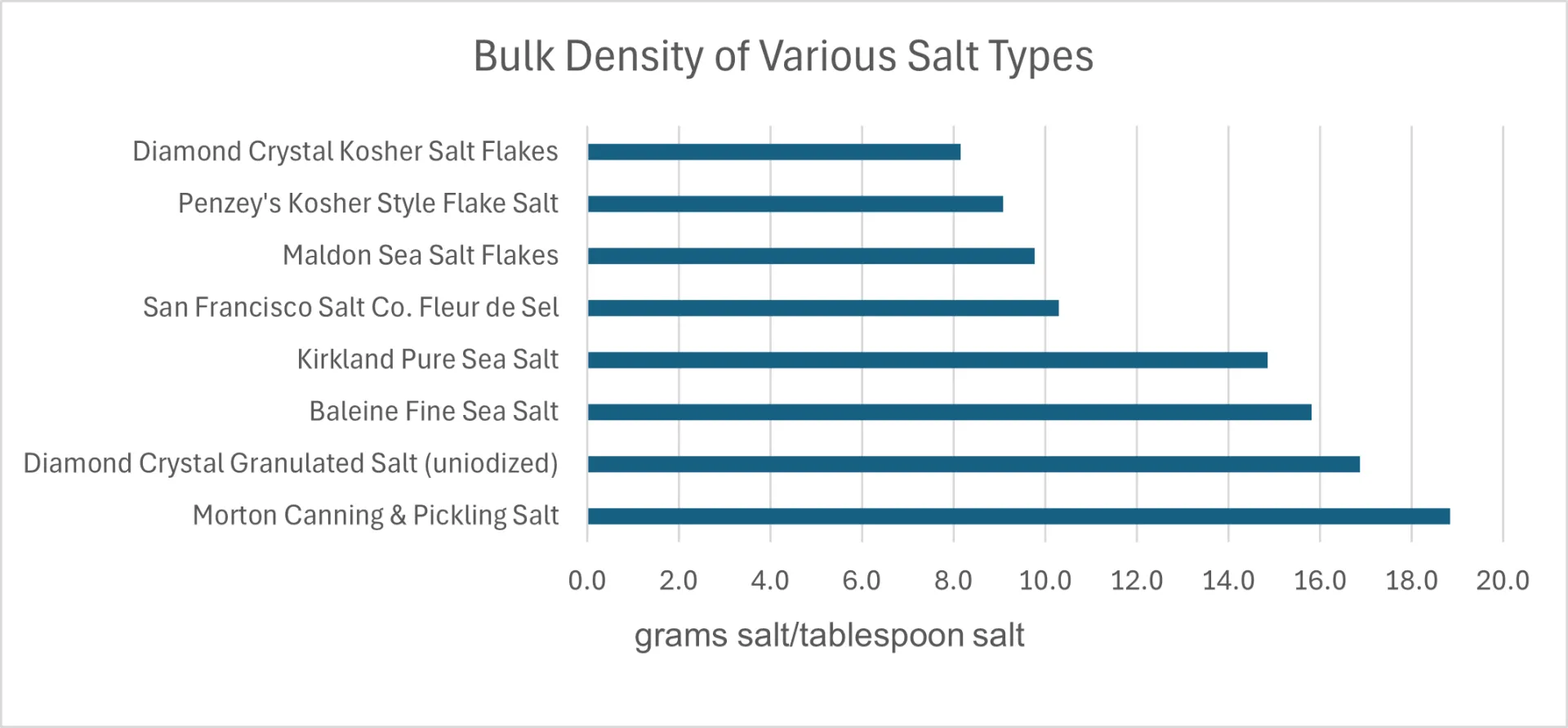
Image credits: Henry Shaw, 2025.
Our salts include two “specialty” items that one would not normally think of using in a ferment: the Maldon Salt and the Fleur de Sel. Both are “finishing” salts, intended to be sprinkled on foods just before they are served or as a top-garnish on baked goods or chocolates. They are both characterized by very large, thin particles, with the Maldon particles having a characteristic “hopper growth” shape (think of a hollow, stepped, Aztec pyramid). As one might expect from the shape of the particles, both these salts came in at the lower end of the range of bulk densities.
Stay tuned for Part 2 of this series of articles on salt, in which I’ll discuss the chemistry of various salts as it related to food preservation… Can I use iodized salt in fermentation? What are “anti-caking agents” and why do I care? What’s the difference between sea salt and regular salt? Why is “Himalayan pink salt” pink and can I use that in preserving?
[Disclaimer: The mention of specific products in this article does not imply endorsement or opposition of these products by the University of California. Product names are only given for informational purposes.]
Extended history...
Some salt is a necessary part of our diet to maintain our electrolyte balance, nerve function, and muscle function. Perhaps more relevant to this article, salt has been used for centuries in food preservation and was highly valued for that purpose. Salt deposits are rarely seen exposed at the surface of the Earth; they rapidly dissolve in rainwater and get washed away. Communities located far from the ocean typically had no local source of salt and relied on trade via long caravans or river routes like the famous Trans-Saharan salt trade or China's salt roads. These inland societies traded valuable goods like furs, grain, or gold (sometimes on one-for-one basis by weight), in exchange for salt. Today, we produce hundreds of millions of tons of salt globally per year by evaporation of seawater and other natural brines, and hard-rock or solution mining of large, underground halite deposits.
